Abstract
Background: First-line conventional chemoimmunotherapy in MCL can be improved. Promising results have been seen with Bruton tyrosine kinase inhibitors (BTKis) in combination with venetoclax (V) and an anti-CD20 monoclonal antibody in patients (pts) with relapsed/refractory or TN MCL. Acalabrutinib (A) is a next-generation, highly selective, covalent BTKi currently approved for relapsed/refractory MCL. We report initial safety and efficacy results of the ongoing, multicenter, open-label phase 1b study of A, V, and rituximab (R) (AVR) in TN MCL.
Methods: TN MCL pts aged ≥18 y with ECOG PS ≤2 were eligible. Starting on cycle 1 day 1, A was administered at 100 mg BID until disease progression or discontinuation for other reasons. R was administered at 375 mg/m 2 on day 1 of each 28-day cycle for 6 cycles, followed by maintenance every other cycle for pts achieving complete response (CR) or partial response (PR), through cycle 24. Starting on cycle 2 day 1, V was administered via an initial 5-wk ramp-up schedule (20, 50, 100, 200, and 400 mg/d) to 400 mg/d, through cycle 25. Dose-limiting toxicity (DLT) was assessed from cycle 2 day 1 to cycle 3 day 28. Primary endpoint was AVR safety. Secondary endpoints were overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS) per Lugano criteria. Positron-emission tomography (PET)/computed tomography (CT) scans were performed after 3 and 6 cycles and to confirm CR at any time. CT scans were performed after 3, 6, 9, and 12 cycles, and then every 6 cycles. Longitudinal minimal residual disease (MRD) was assessed using the clonoSEQ assay in peripheral blood at PR, CR, every 6 cycles post-CR, and treatment end.
Results: 21 pts were enrolled (median age 66 y [range 51-85]; ECOG PS ≤1 20 [95%]; Ann Arbor stage IV disease 19 [90%]; bulky disease >5 cm 7 [33%]; intermediate- and high-risk simplified MCL International Prognostic Index scores 11 [52%] and 4 [19%], respectively; blastoid variant 1 [5%]; and Ki-67 proliferation index ≥50% 3 [14%]). Fifteen (71%) pts had bone marrow (BM) involvement at baseline. As of March 19, 2021, median time on study was 16 mo (range 8-26.2). Median (range) number of cycles administered was 15 (7-27) for A, 13.5 (5-23) for 400 mg daily V, and 12 (6-15) for R. Seventeen (81%) pts remain on study treatment and 4 (19%) have discontinued (progressive disease: n=1; COVID-19 infection: n=3).
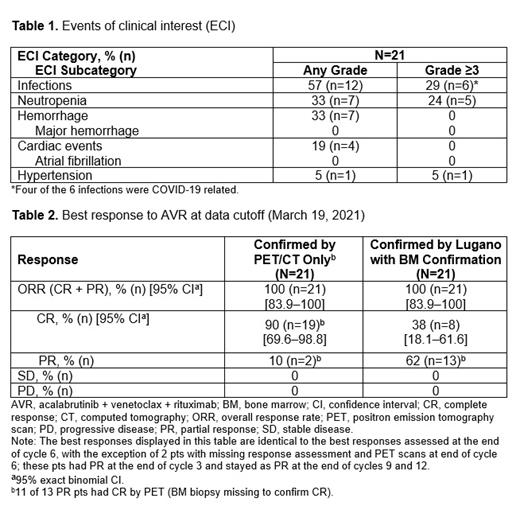
No DLTs were observed; V 400 mg daily after ramp-up was the dose chosen for triple therapy. Most common any-grade AEs in ≥20% of pts were diarrhea (13 [62%]), headache (11 [52%]), fatigue (10 [48%]), neutropenia (6 [29%]), paresthesia (6 [29%]), cough (6 [29%]), dyspnea (6 [29%]), myalgia (5 [24%]), dizziness (5 [24%]), and hypoesthesia (5 [24%]). Grade 3/4 AEs in ≥2 pts were neutropenia (5 [24%]) and pneumonia (2 [10%]). Serious any-grade AEs in ≥2 pts were COVID-19 infection (4 [19%]) and pneumonia (2 [10%]). In the 4 pts with COVID-19 infection, the events led to triple-drug discontinuation and death in 3 pts and to dose holds of A and V and event resolution in 1 pt (all considered unrelated to study treatment). Diarrhea led to V dose reduction in 1 pt. AEs led to dose holds in 12 (57%) pts and were associated with A, V, and R in 52%, 48%, and 14%, respectively. Events of clinical interest are shown in Table 1.
At the end of cycle 6, ORR was 100%, with CR/PR in 90%/10% by PET/CT alone (11 of the 13 CRs by PET/CT lacked BM confirmation); the CR/PR rate by Lugano criteria with BM confirmation was 38%/62% (Table 2). Median DOR was 19 mo (95% CI 17-not estimable [NE]) overall, and not reached when the 3 pts with COVID-19 deaths were censored. Median PFS and OS were not reached. The 1-y PFS and OS rates were 89% (95% CI 62-97) and 95% (95% CI 71-99), respectively. Treating the 3 COVID-19 deaths as censored, the 1-y PFS rate was 93.8% (95% CI 63.2-99.1). Median time to initial response and best response was 2.8 mo. Twelve of 16 (75%) pts with available MRD results at cycle 6 achieved MRD negativity (10 -6), including 6 pts with CR who attained MRD negativity at the time of CR or earlier and continued to be MRD negative at cycle 24. Six pts with PR also achieved MRD negativity, suggesting deeper molecular responses. One pt with PR became MRD positive before having clinical disease progression.
Conclusions: The triple combination of acalabrutinib, venetoclax, and rituximab is well tolerated and provides a 100% clinical response rate and high rate of complete molecular response in TN MCL.
Wang: Loxo Oncology: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Physicians Education Resources (PER): Honoraria; Scripps: Honoraria; InnoCare: Consultancy, Research Funding; Molecular Templates: Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Oncternal: Consultancy, Research Funding; BGICS: Honoraria; DTRM Biopharma (Cayman) Limited: Consultancy; Mumbai Hematology Group: Honoraria; Anticancer Association: Honoraria; Genentech: Consultancy; OMI: Honoraria; Dava Oncology: Honoraria; Epizyme: Consultancy, Honoraria; CAHON: Honoraria; CStone: Consultancy; Bayer Healthcare: Consultancy; Chinese Medical Association: Honoraria; Imedex: Honoraria; Newbridge Pharmaceuticals: Honoraria; Moffit Cancer Center: Honoraria; Clinical Care Options: Honoraria; Miltenyi Biomedicine GmbH: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Research Funding; Hebei Cancer Prevention Federation: Honoraria; Lilly: Research Funding; Celgene: Research Funding; BioInvent: Research Funding; VelosBio: Consultancy, Research Funding; Juno: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; The First Afflicted Hospital of Zhejiang University: Honoraria; Acerta Pharma: Consultancy, Honoraria, Research Funding. Robak: Biogen, Abbvie, Octapharma, Janssen: Honoraria, Other: Advisory board; AstraZeneca, Abbvie, Janssen, Octapharma, Gilead,Oncopeptides AB, Pharmacyclics, Pfizer, GlaxoSmithKline, Biogen: Research Funding; Medical University of Lodz: Current Employment. Maddocks: Karyopharm: Divested equity in a private or publicly-traded company in the past 24 months; KITE: Divested equity in a private or publicly-traded company in the past 24 months; Celgene: Divested equity in a private or publicly-traded company in the past 24 months; ADC Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; Beigene: Divested equity in a private or publicly-traded company in the past 24 months; Merck: Divested equity in a private or publicly-traded company in the past 24 months; Seattle Genetics: Divested equity in a private or publicly-traded company in the past 24 months; Janssen: Divested equity in a private or publicly-traded company in the past 24 months; Novatis: Divested equity in a private or publicly-traded company in the past 24 months; BMS: Divested equity in a private or publicly-traded company in the past 24 months; Morphosys: Divested equity in a private or publicly-traded company in the past 24 months; Pharmacyclics: Divested equity in a private or publicly-traded company in the past 24 months. Phillips: Abbvie: Consultancy, Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy; Bayer: Research Funding; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; KITE: Membership on an entity's Board of Directors or advisory committees; Lymphoma Connect: Honoraria; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Smith: Beigene: Consultancy, Research Funding; Merck Sharp & Dohme Corp: Research Funding; Millenium/Takeda: Consultancy; Incyte: Consultancy; Acerta Pharma BV: Research Funding; Ignyta (spouse): Research Funding; Bayer: Research Funding; AstraZeneca: Consultancy, Research Funding; Karyopharm: Consultancy; ADC Therapeutics: Consultancy; Genentech: Research Funding; Portola Pharmaceuticals: Research Funding; Ayala (spouse): Research Funding; Incyte Corporation: Research Funding; De Novo Biopharma: Research Funding; Bristol Myers Squibb (spouse): Research Funding; KITE pharm: Consultancy. Calvo: AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Wun: AstraZeneca: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Munugalavadla: AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Jurczak: AstraZeneca, BeiGene, Janssen, Loxo Oncology, Sandoz, Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie, AstraZeneca, BeiGene, Celtrion, Celgene, Debbiopharm, Epizyme, Incyte, Janssen, Loxo Oncology, Merck, Mei Pharma, Morphosys, Novo Nordisk, Roche, Sandoz, Takeda, TG Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal